The prevalence of diabetes significantly increased in adults aged 18 years or older from 2001 to 2020.
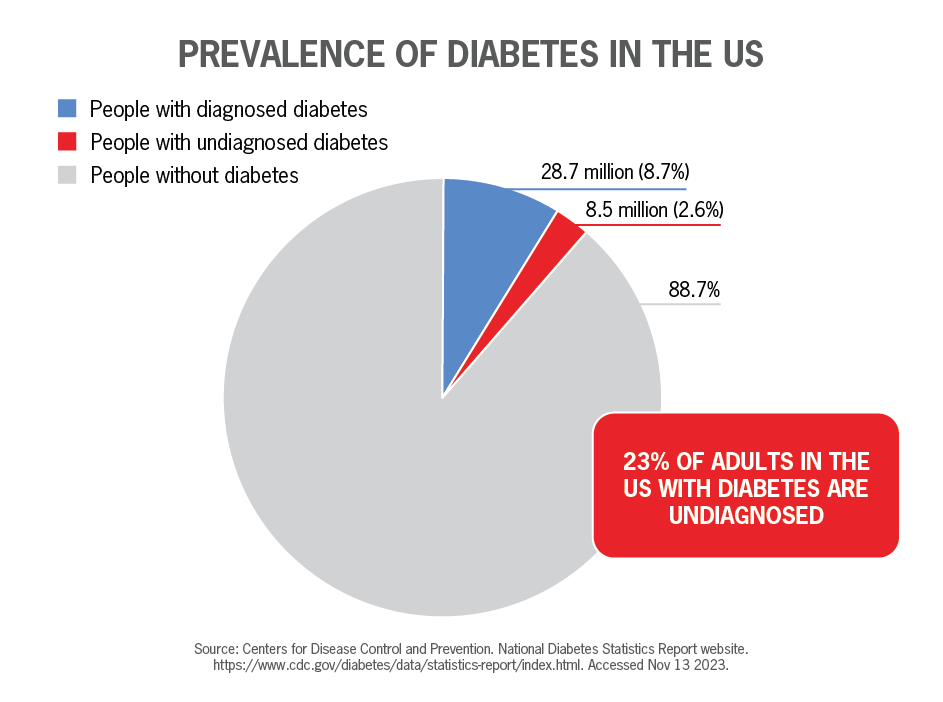
In fact, the Centers for Disease Control and Prevention (CDC) says that approximately 37.3 million Americans have diabetes—which is 11.3% of the US population—with 1.75 million Americans diagnosed with diabetes every year.
To tackle diabetes, some remarkable devices and therapeutics have recently been developed.
Devices
There’s been an explosion of interest in continuous glucose monitoring (CGM) devices, especially those which do not require finger sticks.
An important new entrant is the MiniMed™ 780G CGM system manufactured by Minneapolis, MN-based Medtronic.
According to the manufacturer, the MiniMed 780G system is “designed with SmartGuard™ technology, an advanced algorithm that uses current and past sugar level trends to anticipate insulin needs, adjust insulin delivery, and correct highs automatically” while protecting patients from lows.
The MiniMed 780G insulin pump pairs with the new Guardian™ 4 sensor and integrates proprietary smartphone apps with the Apple iPhone®and certain Samsung Galaxy smartphones.
Biologics
Researchers at Boston, MA-based Vertex have been busy testing a new biologic, VX-880, using stem cells to replace insulin-producing beta cells in people with type 1 diabetes.
In the newest results, 6 out of 6 participants saw improved blood sugar levels and more time-in-target blood-sugar range, with 3 participants able to stop taking insulin altogether.
According to Vertex, VX-880 is an “investigational allogeneic stem cell-derived, fully differentiated, insulin-producing islet cell therapy manufactured using proprietary technology.”
Pharmacotherapies
In August 2023, at the Riley Hospital for Children, patient Colin Ozdemir became the first child in Indiana to receive Tzield® teplizumab, a new therapy that prevents the onset of stage 3 type 1 diabetes by an average of 3 years.
The drug received Federal Drug Administration approval in November 2022. While the treatment is not intended to permanently prevent a diagnosis, the Ozdemir family and diabetes experts say that delaying the disease by at least a couple of years is an incredible advance that improves the quality of life for those at risk. The manufacturer of Tzield® teplizumab is Sanofi.
While diabetes prevalence has been increasing, it is reassuring to know that very promising new devices and treatments continue to emerge.

